Borrelia Burgdorferi: Symptoms and
Treatment. (n.d.). Borrelia Burgdorferi. Borrelia
Burgdorferi: Symptoms and Treatment.
Retrieved from http://borreliaburgdorferi.org/.
Ghiasvand, A. (2011, Nov. 11). Life Cycles
& Reproduction. [Awesome Inc.].
Retrieved from http://borrelia-burgdorferi.blogspot.ca/2011/11/life-cycles-reproduction.html.
Retrieved from http://borrelia-burgdorferi.blogspot.ca/2011/11/life-cycles-reproduction.html.
Hunt, Richard. (2010, April 19).
Parasitology Chapter Seven Part Two Ticks. Microbiology
and Immunology On-line University of South Carolina School of Medicine.
Retrieved from http://pathmicro.med.sc.edu/parasitology/ticks.htm.
Retrieved from http://pathmicro.med.sc.edu/parasitology/ticks.htm.
American Society for Microbiology. (2013,
March 25). Motility is crucial for the infectious life cycle of Borrelia
burgdorferi. American Society for
Microbiology: Infection and Immunity. Retrieved from http://iai.asm.org/content/early/2013/03/19/IAI.01228-12.abstract.
Kung F., Anguita J., & Pai U. (n.d.). Borrelia
Burgdorferi and Tick Proteins Supporting Pathogen Persistence in the Vector. Medscape News. Retrieved from http://www.medscape.com/viewarticle/776862.
Sal M. S., Li C., Motalab. M. A., Shibata
S., Aizawa S., & Charon N. W. (2008, Jan. 11). Borrelia burgdorferi
Uniquely Regulates Its Mobility Genes and Has an Intricate Flagellar Hook-Basal
Body Structure. National Center for
Biotechnology Information, U.S. National Library of Medicine. Retried from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2258876/.
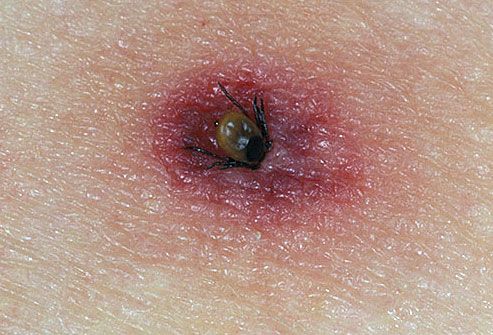
Interactive Medical Media LLC. (n.d.). Tick Bites. [Photograph]. Retrieved from http://www.webmd.com/skin-problems-and-treatments/picture-of-tick-bites.
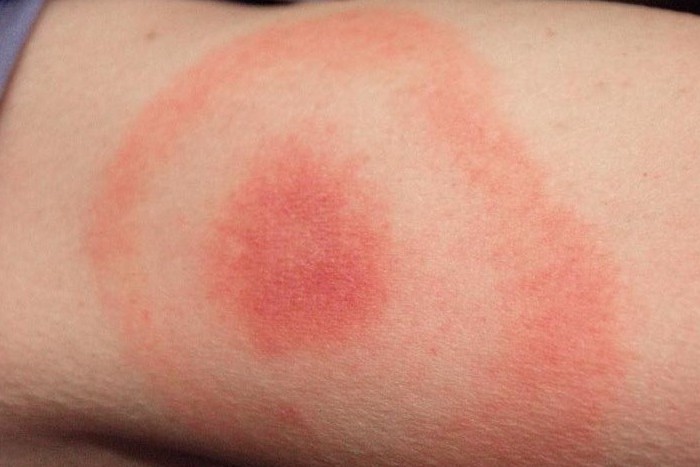
Gathany J. (2010, Dec. 8). Lyme Disease Rash Picture. [Photograph]. Retrieved from http://hardinmd.lib.uiowa.edu/cdc/lymedisease6.html.
VanDyk J. (1996, June 12). Ixodes Scapularis, the Black-Legged or Deer
Tick. [Photograph]. Retrieved from http://www.ent.iastate.edu/imagegallery/ticks/iscapall4wd.html.
Stanek, G. (2012). Lyme borreliosis. [Microscopic Diagram].
Retrieved from http://www.sciencedirect.com/science/article/pii/S0140673611601037.
Retrieved from http://www.sciencedirect.com/science/article/pii/S0140673611601037.
Bacmap Genome Atlas. (n.d.). Borrelia
burgdorferi B31. [Microscopic Photograph]. Retrieved from http://bacmap.wishartlab.com/organisms/19.
Rosa, P. A., Tilly K., & Stewar, P. E. (2005).
Lyme Disease. [Diagram].
Retrieved from http://www.nature.com/nrmicro/journal/v3/n2/box/nrmicro1086_BX1.html.
Biology Exams 4 U. (2013). Difference between prokaryotic and eukaryotic chromosome. [Diagram].
Retreived from http://www.biologyexams4u.com/2012/11/difference-between-prokaryotic-and.html.
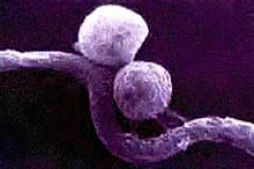
Micro*scope. (2006). Mixed Population. [Microscopic Photograph].
Retrieved from http://starcentral.mbl.edu/microscope/portal.php?pagetitle=assetfactsheet&imageid=755.
Vojdani A., Hebroni F., Raphael Y., Erde J., & Raxien B. (2007). Novel Diagnosis of Lyme Disease: Potential for CAM Intervention. [Diagram].
Retrieved from http://openi.nlm.nih.gov/detailedresult.php?img=2722197_nem138f1&req=4.
Kumaran D., Eswaramoorthy S., Luft B. J., Koide S., Dunn J. J., Lawson C. L., & Swaminathan S. (2001). Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi. [Diagram].
Retrieved from http://www.nature.com/emboj/journal/v20/n5/fig_tab/7593602a_F2.html.
AMI Health. (2010). Lyme Disease Treatment. [Poster].
Retrieved from
http://www.amihealth.com/lyme-disease-treatment.html.
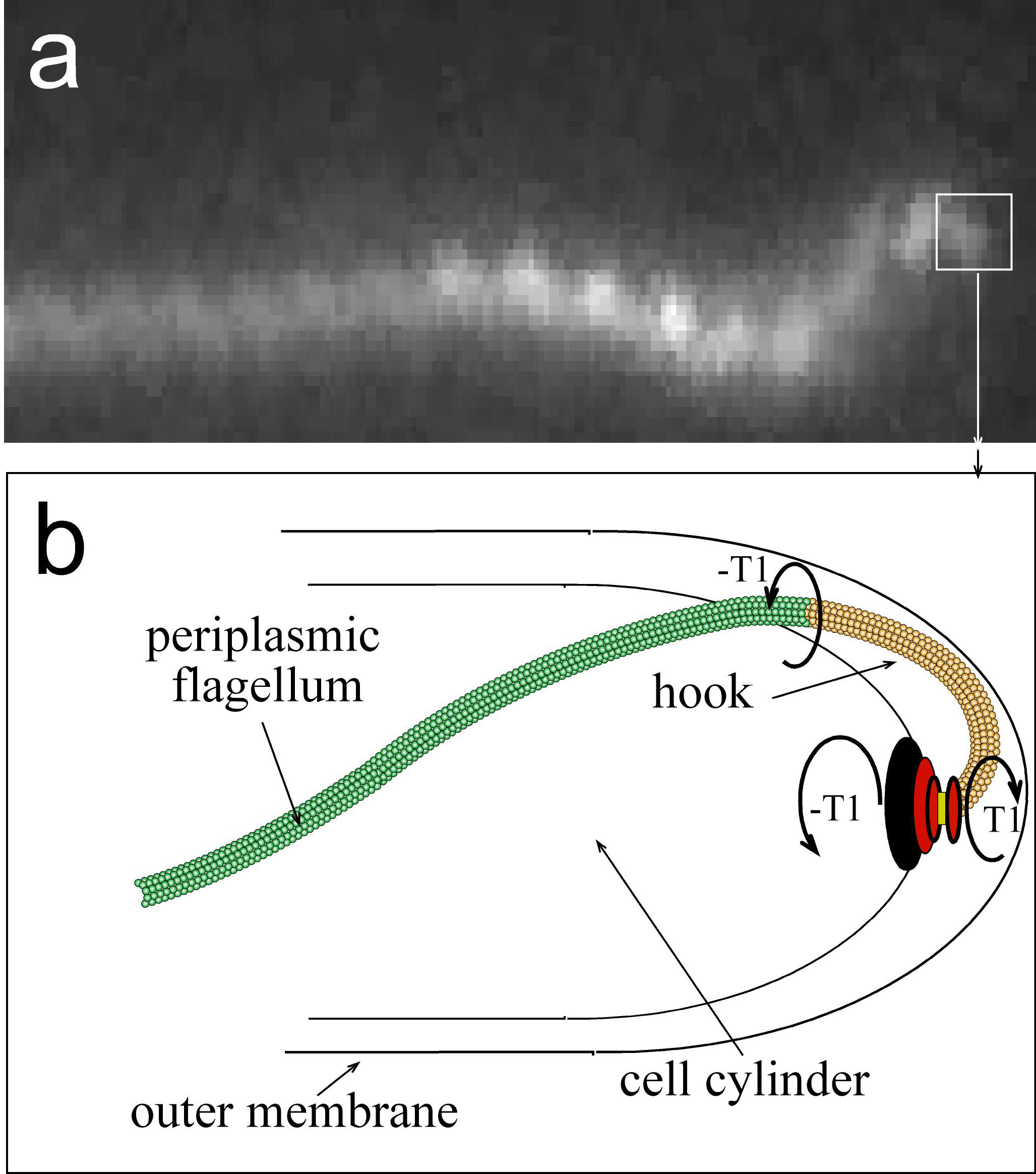
(n.d.) Spirochete Morphology and Motility. [Photograph & Diagram] Retrieved from http://www.physics.arizona.edu/~wolg/research.html.
American Lyme Disease Foundation. (2010). Deer Tick Ecology. [Diagram]. Retrieved from
http://www.aldf.com/deerTickEcology.shtml.
Biology Exams 4 U. (2013). Difference between prokaryotic and eukaryotic chromosome. [Diagram].
Retreived from http://www.biologyexams4u.com/2012/11/difference-between-prokaryotic-and.html.
Micro*scope. (2006). Mixed Population. [Microscopic Photograph].
Retrieved from http://starcentral.mbl.edu/microscope/portal.php?pagetitle=assetfactsheet&imageid=755.
Vojdani A., Hebroni F., Raphael Y., Erde J., & Raxien B. (2007). Novel Diagnosis of Lyme Disease: Potential for CAM Intervention. [Diagram].
Retrieved from http://openi.nlm.nih.gov/detailedresult.php?img=2722197_nem138f1&req=4.
Kumaran D., Eswaramoorthy S., Luft B. J., Koide S., Dunn J. J., Lawson C. L., & Swaminathan S. (2001). Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete, Borrelia burgdorferi. [Diagram].
Retrieved from http://www.nature.com/emboj/journal/v20/n5/fig_tab/7593602a_F2.html.
AMI Health. (2010). Lyme Disease Treatment. [Poster].
Retrieved from
http://www.amihealth.com/lyme-disease-treatment.html.
(n.d.) Spirochete Morphology and Motility. [Photograph & Diagram] Retrieved from http://www.physics.arizona.edu/~wolg/research.html.
American Lyme Disease Foundation. (2010). Deer Tick Ecology. [Diagram]. Retrieved from
http://www.aldf.com/deerTickEcology.shtml.